TECHNOLOGY
Pipeline
Eyedrops for Fuchs Endothelial Corneal Dystrophy
Corneal Endothelial Therapeutic Cell Product
Background
The cornea and corneal endothelium
The cornea is located at the anterior surface of the eyeball. It is a transparent tissue that can be compared to a car windshield. The cornea must be transparent to allow outside light into the eye, and this transparency is maintained by the corneal endothelium, which is located on the back side of the cornea (inside the eye).
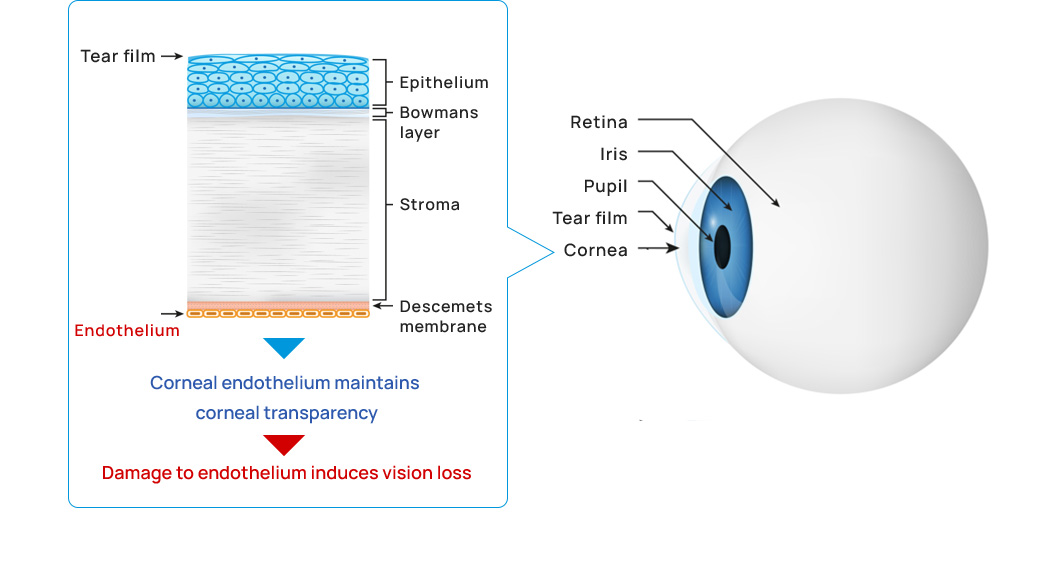
Corneal endothelial diseases
When the corneal endothelium is damaged by disease, eye surgery, or injury, the cornea becomes white and cloudy, and vision is reduced. This condition is called corneal endothelial decompensation or bullous keratopathy. The main causes of corneal endothelial decompensation are Fuchs endothelial corneal dystrophy, cataract surgeries, other eye surgeries, and rejection after corneal transplantation. Fuchs endothelial corneal dystrophy is responsible for about 40% of all corneal transplants worldwide.
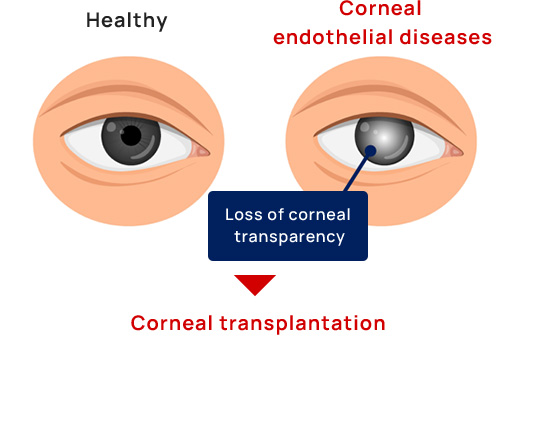
Current treatment of corneal endothelial diseases
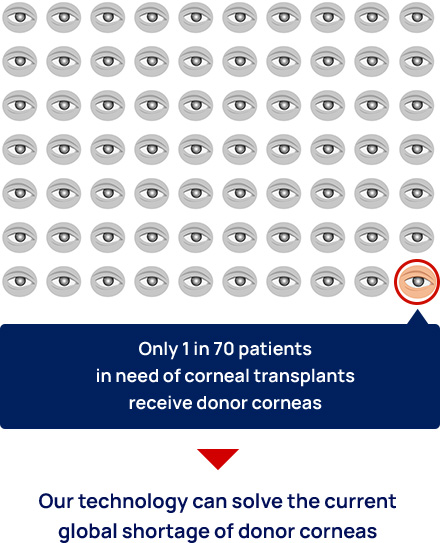
Currently, the only treatment for corneal endothelial decompensation is corneal transplantation, in which the cornea is donated as an organ from a deceased person. Donor corneas are in such a short supply around the world that only one in 70 patients worldwide who need a corneal transplant is able to receive one. The number of corneal transplantations needed for the treatment of corneal endothelial decompensation has been increasing for two decades.
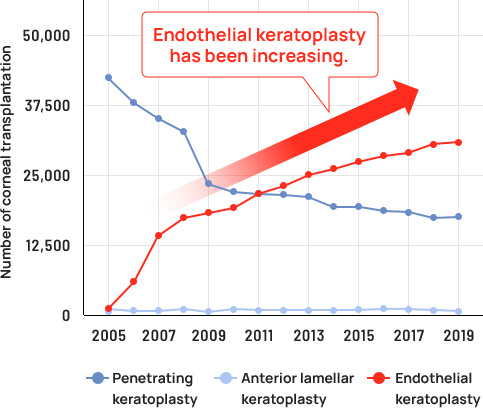
Our
Solution
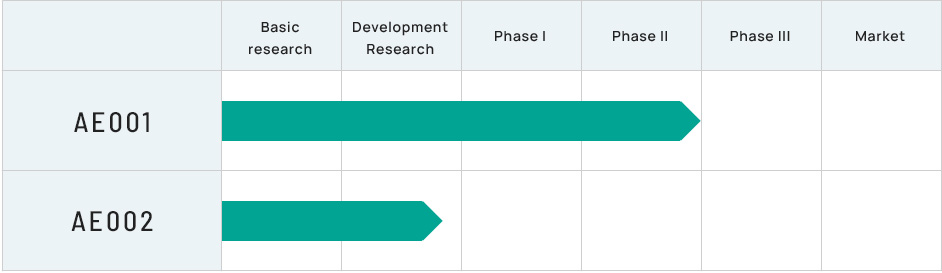
Product candidate for the treatment of Fuchs endothelial corneal dystrophy(development codes: AE001 and AE002)
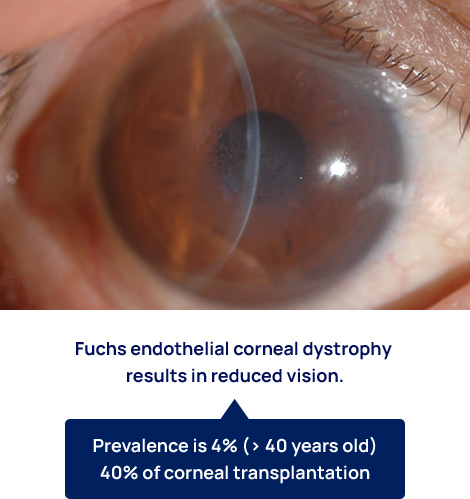
Fuchs endothelial corneal dystrophy
Fuchs endothelial corneal dystrophy affects 4% of persons over the age of 40. The early stages of the disease have no subjective symptoms, but as the disease progresses, patients experience glare and vision loss. As the disease progresses further, eye pain and tearing may occur, and the cornea may become cloudy.
This disease is often diagnosed at a relatively early stage when vision is good, but because no cure is available, corneal transplants are performed only after vision has decreased severely. Fuchs endothelial corneal dystrophy is the leading cause of corneal transplantation worldwide, and the development of alternative treatments for corneal transplantation is highly anticipated.
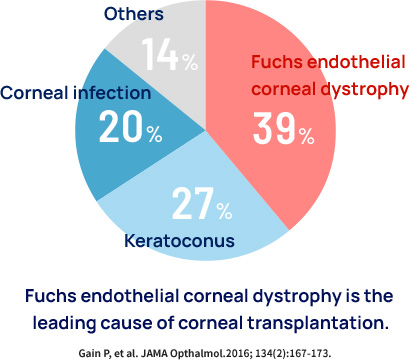
Discovery of therapeutic drug candidates
Professors Noriko Koizumi and Naoki Okumura at Doshisha University, both co-founders of ActualEyes, Inc., have been working to elucidate the mechanism of Fuchs endothelial corneal dystrophy and to develop pharmaceutical therapies. Corneal endothelial cells were obtained from the patients with Fuchs endothelial corneal dystrophy during corneal transplantation. The corneal endothelial cells were first cultured as a disease model of Fuchs endothelial corneal dystrophy. Using this cell model, Drs. Koizumi and Okumura investigated potential drugs for the treatment of Fuchs endothelial corneal dystrophy. Their drug screening identified sirolimus as a drug that dramatically stops the death of corneal endothelial cells. ActualEyes, Inc. has obtained an exclusive license from Doshisha University to use sirolimus as a treatment for Fuchs endothelial corneal dystrophy and is developing it as a therapeutic agent.
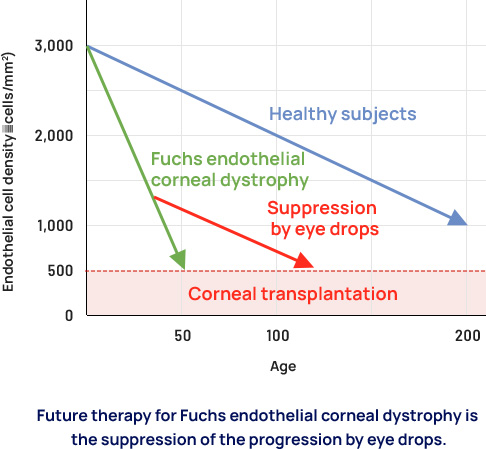
ActualEyes, Inc. is also developing AE002 (development code: AE002), which is expected to be an effective treatment for Fuchs endothelial corneal dystrophy through a different mechanism of action. Once these eye drops are launched, the future need for corneal transplants due to Fuchs endothelial corneal dystrophy may be drastically reduced. We expect that these drops will change the treatment of corneal endothelial disease.
- Sirolimus (development code: AE001)
- In November 2021, ActualEyes, Inc. entered into a Phase II clinical trial (Phase IIa / POC) with Santen Pharmaceutical Co., Ltd. for a sirolimus ophthalmic solution (Santen development code: STN1010904, ActualEyes, Inc. development code: AE001) for the treatment of Fuchs endothelial corneal dystrophy. The clinical trial was started in the U.S. in June 2022. If the clinical trial confirms sirolimus as a safe and effective drug, the world's first treatment for Fuchs endothelial corneal endothelial dystrophy will be launched.
- AE002 (development code: AE002)
- AE002 is another product candidate with expected efficacy in preventing the progression of Fuchs endothelial corneal dystrophy. We are preparing clinical trials for AE002.
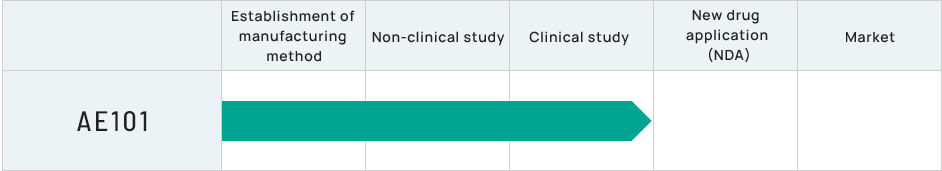
Regenerative medicine candidate for corneal endothelial diseases (development code: AE101)
Successful clinical research on corneal endothelial regenerative medicine
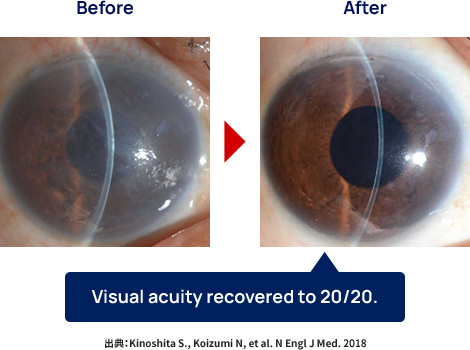
Noriko Koizumi and Naoki Okumura, founding members of ActualEyes, Inc., have devoted their efforts to developing regenerative therapy for corneal endothelial diseases. They have succeeded in the extremely difficult task of culturing corneal endothelial cells for clinical use. Furthermore, they discovered that injection of cultured corneal endothelial cells, together with a drug called ROCK inhibitor, into the eye can be an alternative to conventional corneal transplantation. These technologies were transferred to the Kyoto Prefectural University of Medicine, and the world's first-in-human study of cell injection therapy was initiated in December 2013. Since then, more than 50 patients have been successfully treated, and the efficacy and safety of this treatment have been confirmed. The fact that patients who needed corneal transplants have regained their sight through regenerative medicine has been a remarkable achievement. This regenerative medicine involves injecting cells into the eye using an injection needle, and the treatment is less burdensome to the patient than a corneal transplant, as it is completed in less than 10 minutes. Furthermore, the resulting reconstructed cornea is nearly anatomically normal, so the restoration of vision is considered ideal.
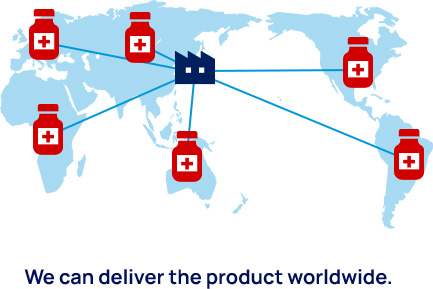
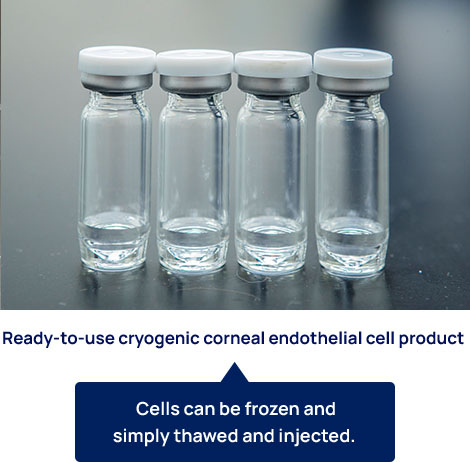
Ready-to-use cryogenic corneal endothelial cell product
The success of the clinical research conducted at the Kyoto Prefectural University of Medicine Hospital has increased the expectations for successful regenerative medicine for corneal endothelial disorders worldwide. ActualEyes, Inc. has developed a formulation of cultured cells that can be frozen and then simply thawed and injected when needed. Once commercialized, the product will be delivered worldwide for the treatment of patients on an as-needed basis. ActualEyes, Inc. is now preparing clinical trials in Japan and China (in collaboration with Arctic Vision, Inc.).
A solution to the global donor cornea shortage
We have successfully developed a mass culture system for corneal endothelial cells. At present, we can culture the corneal endothelial cells obtained from a single donor cornea to produce a product that can treat 50–80 people. At present, only 1 in 70 patients in need of corneal transplants actually receive donor corneas; therefore, our technology could theoretically solve the current global shortage of donor corneas. Our cell products can be frozen and stored for delivery to patients around the world for treatment when they need it.